
The cycle was developed by contribution from both of them in the year 1919. Thus, ΔH ⦵ 1st E.A. (Cl 2) = −347.The Born-Haber process is named after the two famous German Scientists Max Born and Fritz Haber. ΔH ⦵ dissociation L.E= –ΔH ⦵ f(BaCl 2) +ΔH ⦵ at (Ba )+ΔH ⦵ 1st I.E. Use the data below to calculate a value for the electron affinity of chlorine.Ĭl 2(g) → 2Cl(g) +244 ( ΔH ⦵ B.E. Use the data below to determine the standard enthalpy change for the formation of one mole of calcium fluoride in its standard state, from its constituent elements in their standard states, under standard conditions.į 2(g) → 2F(g) +158 ( ΔH ⦵ B.E. Q1. Methanol can be regarded as a carbon-neutral fuel because it can be synthesised from carbon dioxide and hydrogen. Calculate the standard enthalpy change for this reaction using the data given below.
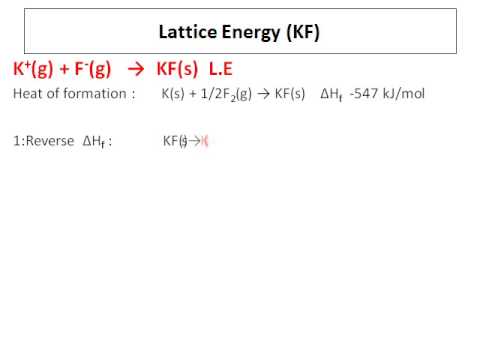
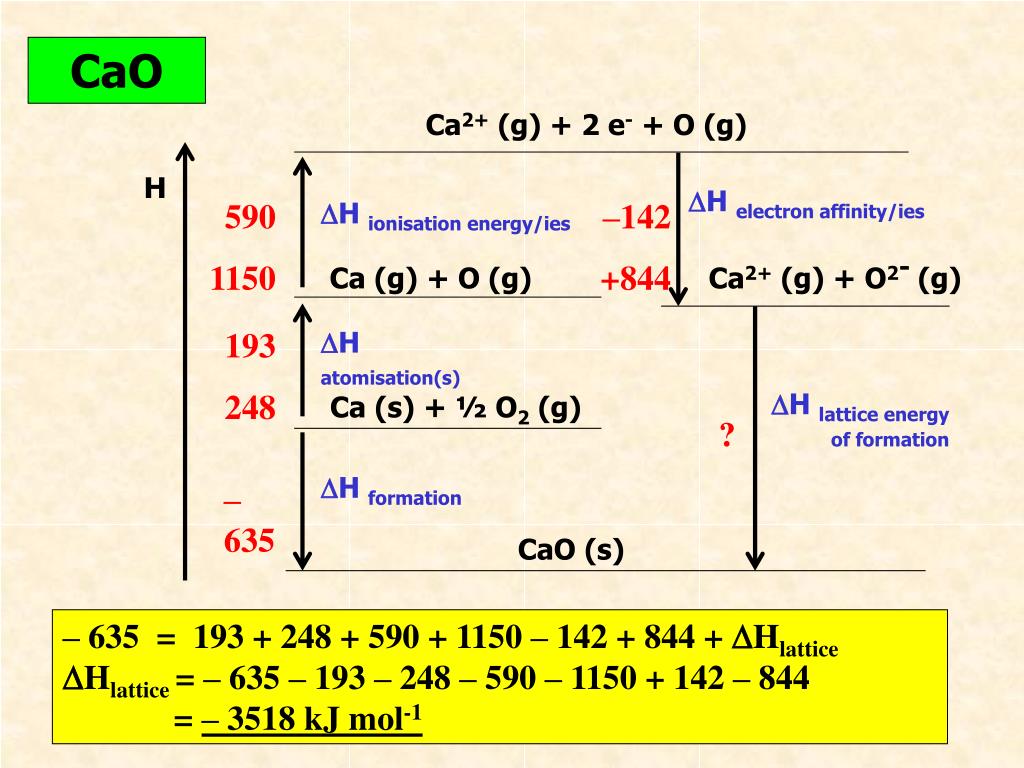
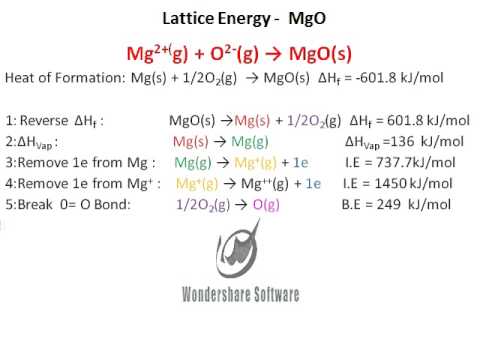
Bond Enthalpy (O=O)Ĥ.Calculating the lattice dissociation enthalpy of calcium sulfide. ΔH ⦵ at Atomisation ½O 2 to form one mole O atoms = ½ × ΔH ⦵ B.E. Bond Enthalpy (Cl−Cl)Ģ.Calculating the lattice dissociation enthalpy of silver chloride ΔH ⦵ at Atomisation ❜l 2 to form one mole Cl atoms = ½ × ΔH ⦵ B.E. Classification, variation, food webs and pyramidsġ.Calculating the lattice dissociation enthalpy of sodium chloride.Combustion reactions and impact on climate.Atoms elements compounds and mixtures (interactive).
B1.6 Waste materials from plants and animals.RSC Learn Chemistry Classic Chemistry Experiments.RSC Classic Chemistry Experiments (1995).Practical Chemistry (Nuffield Foundation/RSC).3.15 Nuclear magnetic resonance spectroscopy.3.14 Organic synthesis and analysis (A2).2.6 Reactions of ions in aqueous solution.2.4 Properties of Period 3 elements and their oxides.1.11 Electrode potentials and electrochemical cells (Redox A2).1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2).3.6 Organic analysis (AS): analytical techniques.1.7 Oxidation reduction equations (Redox AS).1.6 Chemical equilibria and Le Chatelier’s principle.C3.5 Production of ammonia (an example of a reversible reaction).C3.4 Further analysis and quantitative chemistry.C3.3 Calculating and explaining energy change.C2.5 Exothermic and endothermic reactions.C2.3 Atomic structure, analysis and quantitative chemistry.C1.7 Changes in the Earth and its atmopshere.C1.5 Other useful substances from crude oil.


 0 kommentar(er)
0 kommentar(er)
